Introduction: Anemia is a hallmark feature of myelofibrosis (MF) and causes many patients (pts) to require red blood cell transfusions. Both anemia and transfusion dependency have been associated with decreased survival in pts with MF. The purpose of this study was to assess the relationship between anemia severity and transfusion dependency on overall survival (OS) among pts with MF in the Medicare population.
Methods: This retrospective cohort study used the Medicare fee-for-service database to identify pts with a diagnosis of MF between January 1, 2012, and December 31, 2020. Study inclusion criteria were as follows: (1) ≥1 inpatient or ≥2 outpatient claims for MF (on separate dates <90 days apart; the earliest appearance of MF was set as the MF diagnostic index date [MFDID]); (2) 12 months of continuous enrollment preceding (baseline) and 6 months of continuous enrollment following (follow-up) the MFDID; (3) absence of Janus kinase inhibitor therapy during the 12-month baseline period; (4) absence of polycythemia vera, essential thrombocythemia, or another primary cancer other than basal and squamous cell skin carcinoma during the baseline period; and (5) absence of participation in a clinical trial.
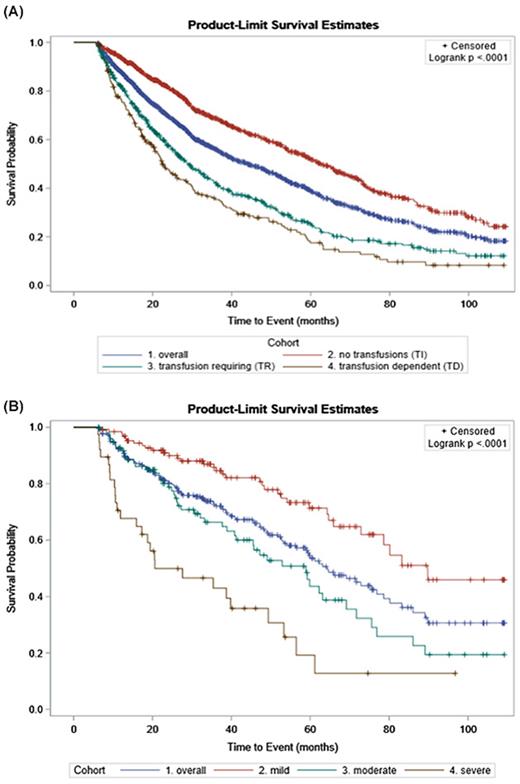
Transfusions were assessed over a 180-day landmark period following anemia index date (AID; set to the earliest evidence of anemia, unless it occurred during the 100 days prior to the MFDID, in which the AID was set to the MFDID), and pts were stratified into 3 subcohorts: transfusion independent (TI; no transfusions), transfusion dependent (TD; ≥6 transfusion claims on 6 separate days over any 12-week window), or transfusion requiring (TR; received transfusion claims but did not meet TD criteria). A subset of pts were identified as having anemia based on medical claims from the Medicare fee-for-service database or laboratory test results from the Prognos Biomarker and Laboratory Database ≤100 days before and any time after the MFDID, with severity subcohorts graded based on hemoglobin levels: mild (10 to <12 g/dL), moderate (8 to <10 g/dL), or severe (<8 g/dL) disease. OS from the MFDID was assessed for all pts using Kaplan-Meier analyses, with log-rank tests used to determine statistically significant differences among the transfusion dependency and anemia severity subcohorts.
Results: A total of 1749 pts qualified for the transfusion cohorts: 980 were TI, 559 were TR, and 210 were TD. Mean age was 74.8 years, 47.9% were male, and 81.7% were White. Results of Kaplan-Meier analyses showed a median OS of 44.1 months (95% CI, 39.8-48.5 months) for the overall transfusion sample and 62.6 months (95% CI, 57.8-68.2 months) for the TI cohort, 29.0 months (95% CI, 25.9-32.9 months) for the TR cohort, and 22.6 months (95% CI, 20.1-28.1 months) for the TD cohort ( P<.001) (Figure A).
A total of 265 pts with corresponding laboratory testing data qualified for the anemia cohorts: 128 were mild, 99 were moderate, and 38 were severe. Mean age was 73.6 years, 47.5% were male, and 79.6% were White. Results of Kaplan-Meier analyses showed a median OS of 64.3 months (95% CI, 56.5-76.9 months) for the overall anemia sample and 89.8 months (95% CI, 72.9 months-not calculable) for the mild cohort, 58.9 months (95% CI, 40.8-69.1 months) for the moderate cohort, and 20.6 months (95% CI, 15.9-49.4 months) for the severe cohort ( P<.001) (Figure B).
Conclusions: These results demonstrate that dependency on transfusions, increasing transfusion requirements, and greater anemia severity are each negative prognostic factors for OS among Medicare fee-for-service pts with diagnosed MF. Therapies that reduce transfusion dependency and anemia severity may aid in improving health outcomes and prolong survival in pts with MF.
Figure. Kaplan-Meier Curves of Overall Survival in Transfusion (A) and Anemia (B) Subcohorts, Calculated from the Myelofibrosis Diagnostic Index Date
Disclosures
Gerds:AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, GlaxoSmithKline, Kartos, Novartis, PharmaEssentia, Sierra Oncology: Consultancy; Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, Kratos Pharmaceuticals: Research Funding. Mesa:Genetech: Research Funding; Promedior: Research Funding; CTI BioPharma., a Sobi Company: Research Funding; Mays Cancer Center: Research Funding; Sierra Onc: Consultancy; Samus: Research Funding; Novartis: Consultancy; Abbvie: Research Funding; Celgene: Research Funding; Constellation: Consultancy, Research Funding; Incyte: Research Funding; LaJolla Pharma: Consultancy; NCI: Research Funding. Tkacz:Inovalon: Current Employment; GSK: Consultancy. Moore-Schiltz:GSK: Other: Employee of Inovalon contracted by GSK to conduct the analysis on this abstract . Schinkel:Sanofi: Consultancy; GSK: Consultancy; Inovalon: Current Employment. Phiri:GSK: Current Employment, Other: Stock Holder. Liu:GSK: Current Employment. Gorsh:GSK: Ended employment in the past 24 months, Other: stock shareholder.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal